Influence of RAAS on the Metabolic Syndrome
Group Members
Prof. Dr. Walter Raasch, Ines Stölting, Laura Beckmann, Laura Nickel, Marco Freschi, Tobias Klersy, Leonie Achner, Luis Hernandez Torres, Alexandra Schmalohr
Research Interests
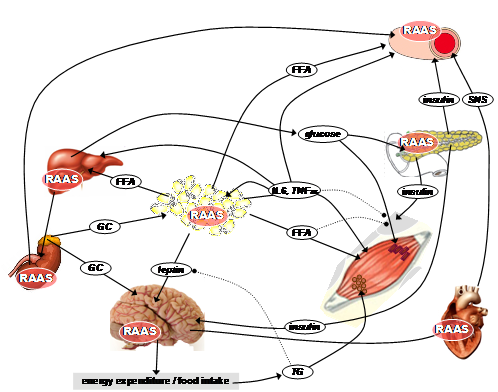
In recent years, metabolic syndrome (MetS) has evolved into a problem of epidemic proportions in Western countries and associates obesity with numerous other abnormalities, including alterations in glucose metabolism, dyslipidemia and hypertension. Accumulating evidence suggests an activation of the renin-angiotensin-aldosterone-system (RAAS) in patients with MetS. Main therapeutic approaches targeting the RAAS are 1.) blocking the conversion of angiotensin I into its bioactive metabolite angiotensin II by the inhibiting the enzyme ACE and 2.) antagonising the (patho-)physiological effects of angiotensin II in various organs by blocking the angiotensin II AT1-receptors. Inhibition of the RAAS represents not only one established approach for lowering high blood pressure but also for treating other cardinal symptoms of MetS. Findings of our group support the broad efficacy of AT1-receptor blockers (ARBs) and ACE-inhibitors on hypertension, glucose utilization and the reactivity of the hypothalamus-pituitary-adrenal (HPA)-axis. Blocking the RAAS and thereby decreasing in the reactivity of the HPA-axis has been found by our group to be one mechanism for explaining the reduced incidence of type 2 diabetes We additionally focused on antiobese actions of ARBs, in particular showing that reduction of body weight after AT1-blockade is related to a downregulation of orexigenic hypothalamic peptides, a reduction of food intake and an increase in energy expenditure. These antiobese effects of ARBs occurs 1.) only after high-dosing, 2.) independently on its antihypertensive potency and 3.) in a manner dependent on an intact leptin signaling. Leptin itself is a hormone which is secreted from fat cells in proportion to body-fat and function as an ‘adiposity signal’ that conveys information to the brain, thus creating a complex network of neural circuits which ultimately elaborate an individual response to a meal. Current projects address the questions whether:
1. the leptin sensitivity is enhanced by ARBs for promoting antiobese effects?;
2. PPARgamma stimulation by ARBs improve glucose utilization in vivo?;
3. the intake of palatable food is altered by ARBs?; and
4. pancreatic glucagon is regulated by ARBs thereby influencing glucose homeostasis.

- Forschung
- Bridging Brain Barriers
- Cellular Electrophysiology
- Cerebral Perfusion and Metabolism
- Influence of RAAS on the Metabolic Syndrome
- Interaction between Tanycytes and the hormone axes
- Neuropeptides and Energy Homeostasis
- Neuroplasticity and Rhythms
- Pharmacovigilance
- WATCH group
- STED microscopy
- Publications
- Memberships
- Seminars
- Awards
- Technologies/Resources
