Associated workgroup: Cellular Electrophysiology
Group members
Prof. Dr. Enrico Leipold, Samuel Kühs, Christina Stanke, Victoria Kemper, Ann-Kathrin Hellberg
Research Interests
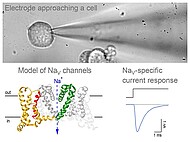
Ion channels are macromolecular protein complexes which from pores across cell membranes to permit controlled movement of inorganic ions into and out of cells. The channel pores open and close in response to specific signals which link channel activity to various physiological functions including signal transmission between cells and metabolism. Voltage-gated sodium channels (NaV channels) are unique among the diverse family of ion channel proteins because they trigger action potentials - a basic feature of excitable cells and the basis for rapid electrical communication along and between neurons and myocytes.
We use a combination of electrophysiological techniques (patch-clamp) and molecular biology methods to study the involvement of NaV channels in human diseases such as peripheral pain disorders and epilepsy. To better understand the disease mechanisms we investigate malfunctions of the channel proteins caused by mutations in the channel-encoding genes of affected patients and we analyze the impact of the mutations on the electrical properties of affected neurons.
Furthermore, we study the interaction between the channel proteins and specific neurotoxins from venomous animals (e.g. marine cone snails, scorpions) because some neurotoxins are valuable tools for analyzing and understanding the basic principles of channel function.
Collaborations
- Prof. Dr. Stefan H. Heinemann, University Jena, Germany
- Prof. Dr. Ingo Kurth, RWTH Aachen University, Germany
- Prof. Dr. Diana Imhof, University Bonn, Germany
- PD Dr. Robert Blum, University Wuerzburg, Germany
Selected Publications
Kuehs S., Teege L., Hellberg AK., Stanke C., Haag N., Kurth I., Blum R., Nau C., Leipold E. (2022) Isolation and transfection of myenteric neurons from mice for patch-clamp applications. Front. Mol. Neurosci., Sec. Pain Mechanisms and Modulators DOI: 10.3389/fnmol.2022.1076
Mohid SA, Biswas K, Won T, Mallela LS, Gucchait A, Butzke L, Sarkar R, Barkham T, Reif B, Leipold E, Roy S, Misra AK, Lakshminarayanan R, Lee D, Bhunia A. Structural insights into the interaction of antifungal peptides and ergosterol containing fungal membrane. Biochimica Et Biophysica Acta. Biomembranes. 183996. PMID 35753394 DOI: 10.1016/j.bbamem.2022.183996
Rakovic A., Voß D., Vulinovic F., Meier B., Hellberg A.K., Nau C., Klein C., Leipold E. (2022) Electrophysiological properties of induced pluripotent stem cell-derived midbrain dopaminergic neurons correlate with expression of tyrosine hydroxylase. Front Cell Neursci DOI: 10.3389/fncel.2022.817198
Leipold E., Hanson-Kahn A., Frick M., Gong P., Bernstein J.A., Voigt M., Katona I., Goral R.O., Altmüller J., Nürnberg P., Weis J., Hübner C.A., Heinemann S.H., Kurth I. (2015) Cold-aggravated pain in humans caused by a hyperactive NaV1.9 channel mutant. Nat Commun 6: 10049.
Leipold E., Liebmann L., Korenke G.C., Heinrich T., Giesselmann S., Baets J., Ebbinghaus M., Goral R.O., Stödberg T., Hennings J.C., Bergmann M., Altmüller J., Thiele H., Wetzel A., Nürnberg P., Timmerman V., De Jonghe P., Blum R., Schaible H.G., Weis J., Heinemann S.H., Hübner C.A., Kurth I. (2013) A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 45: 1399−1404.

- Forschung
- Bridging Brain Barriers
- Cellular Electrophysiology
- Cerebral Perfusion and Metabolism
- Influence of RAAS on the Metabolic Syndrome
- Interaction between Tanycytes and the hormone axes
- Neuropeptides and Energy Homeostasis
- Neuroplasticity and Rhythms
- Pharmacovigilance
- WATCH group
- STED microscopy
- Publications
- Memberships
- Seminars
- Awards
- Technologies/Resources